Blogs & News
A Bold Vision of the Future: Aridhia Attends J.P. Morgan Healthcare Conference & CERSI Summit
Chief Data Officer, Amanda Borens, has been travelling recently, attending the J.P. Morgan Healthcare Conference and the CERSI Summit in San Francisco and taking the opportunity to meet up with valued partner and customer, Quantum Leap Healthcare Collaborative.
Read on to find out more about her trip, what commissioners of the FDA really think about text-based review submissions and how Quantum Leap Healthcare are using the Aridhia DRE to accelerate I-SPY2 trial pathologists’ whole slide images review.
Highlights from San Francisco
It was an inspiring week of healthcare meetings with customers, colleagues past and present, and many new connections. I was honored to represent Aridhia Informatics and kicked off J.P. Morgan Healthcare Conference week with the all-day CERSI Summit on Sunday, followed by dinners, lunches, meetings, and a reception with our esteemed customers at Quantum Leap Healthcare Collaborative (QLHC).
CERSI Summit
The theme for the 5th annual meeting was “A Bold Vision for the Future of Regulatory Science” and it incorporated a good mix of perspectives from academic and pharmaceutical researchers and regulatory agents. For me, there were some hopeful remarks about innovations in regulatory processes and drug developments in the pipeline. Memorable highlights:
FDA Chiefs Chat session included past (Scott Gottlieb, Janet Woodcock, Mark MCClellan) and current (Robert Califf FDA Commissioners discussing ongoing challenges, areas for improvement, and messages of hopefulness.

FDA Chiefs Chat Session
• When Scott Gottlieb rhetorically questioned why regulatory review is not happening in the cloud today – now – Janet Woodcock chimed in and described narrative, text-based submissions as ‘useless’ to loud audience applause. The panel, including moderator (Takeda’s President of R&D) Andy Plump seemed to be in complete agreement. Aridhia continues to advocate for data-driven, real-time regulatory reviews and submissions, and I personally look forward to the impact that cloud-based reviews will have on global drug availability.
• Closing predictions for the year ahead included enthusiasm for weight loss therapies, especially GLP-1 agonists, as a solution for obesity and its downstream comorbidities, and a promise for new therapies for treating the brain, especially promising new mechanisms of action to treat brain disorders and mental health issues.
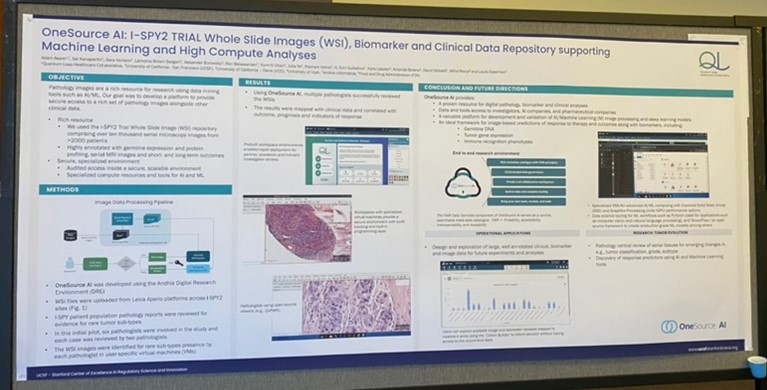
A look at the I-SPY2 Trial’s work within the DRE.
• I was especially grateful for the opportunity to discuss our role in helping I-SPY 2 pathologists review whole slide images inside the Aridhia Digital Research Environment (DRE), and to share de-identified images and annotations with outside researchers by providing secure, audited access in the DRE. As a data scientist of many years, I’ve become immune to the overhyped promises of AI, but I truly believe this novel dataset will help us all learn so much more about breast cancer. Contact us if you’d like to reach QLHC and apply for access to their Aridhia DRE-based platform.
Quantum Leap and I-SPY Collaborators
At the end of a flurry of working meals and meetings, the buzz of J.P. Morgan week in San Francisco really hit me at the offices of Quantum Leap Healthcare Collaborative. We all want access to better prevention, better treatments, and better outcomes for ourselves and our loved ones when cancer is in the picture.

Sara Venters (UCSF) and Amanda Borens (Aridhia)
The atmosphere of investing in new medical products was palpable when I stopped for coffee before leaving for the QLHC reception, and I was reminded why that means so much more than money when I met with several members of the I-SPY trials teams, UCSF and QLHC staff, and startups with medical products in trials. The pace of discovery is dizzying!
The energy and dedication from the people doing the work is very rewarding to see. I was able to thank a scientist who was part of the team that developed Herceptin for her part in saving my sister’s life. A startup hopeful team may have a candidate for treating triple-negative breast cancers, and though I know that it is too early to rely on that drug being successful, I was reminded that the stereotype of ‘evil pharmaceutical companies’ is antithetical to the feeling I get from scientists working so hard to save lives.
On my way home, as I weaved through airport lounges and fished out my meds at the sound of my phone reminder, I had a moment of personal gratitude for the opportunity to remove obstacles and empower people like those on the I-SPY teams to do the good work. Aridhia will continue to focus on technology solutions so that researchers, clinicians, and regulatory agents can focus on saving lives.
January 12, 2024
Amanda Borens
Amanda (M.S.) joined Aridhia in 2022 and serves as Chief Data Officer. She is a data science leader with 25+ years’ experience across public and private sectors, including academia, healthcare, and biotech. Amanda has a unique breadth of life science data curation and analysis experience, having led technical teams in all phases of the FDA-regulated medical software development lifecycle for customers on three continents as well as in medical device development where her team achieved FDA clearance and CE Mark from EMA. This breadth of experience helps her provide innovative data solutions to solve scientific problems while respecting global regulatory requirements.
