Blogs & News
A DRE as a Hub for Training in Quantitative Sciences
As a former academic and still a member of the teaching faculty at the University of Pennsylvania, I can vouch for the benefit of practice problem solving to reinforce didactic teaching lessons. Particularly when teaching various quantitative sciences where problem solving in general is essential. With that in mind, Aridhia recently was invited to support the R25 educational grant with several renowned pediatric clinical pharmacologists in need of an environment to provide a learning environment to their K12 and T32 clinical pharmacology trainees. Clinical Pharmacology is a broad and multifaceted discipline with recent emphasis on various modelling and simulation techniques shown to be a valued complementary skill. Specific skills such as PK/PD modelling, pharmacometrics and data science analytics represent training areas requiring math, statistics and programming expertise.
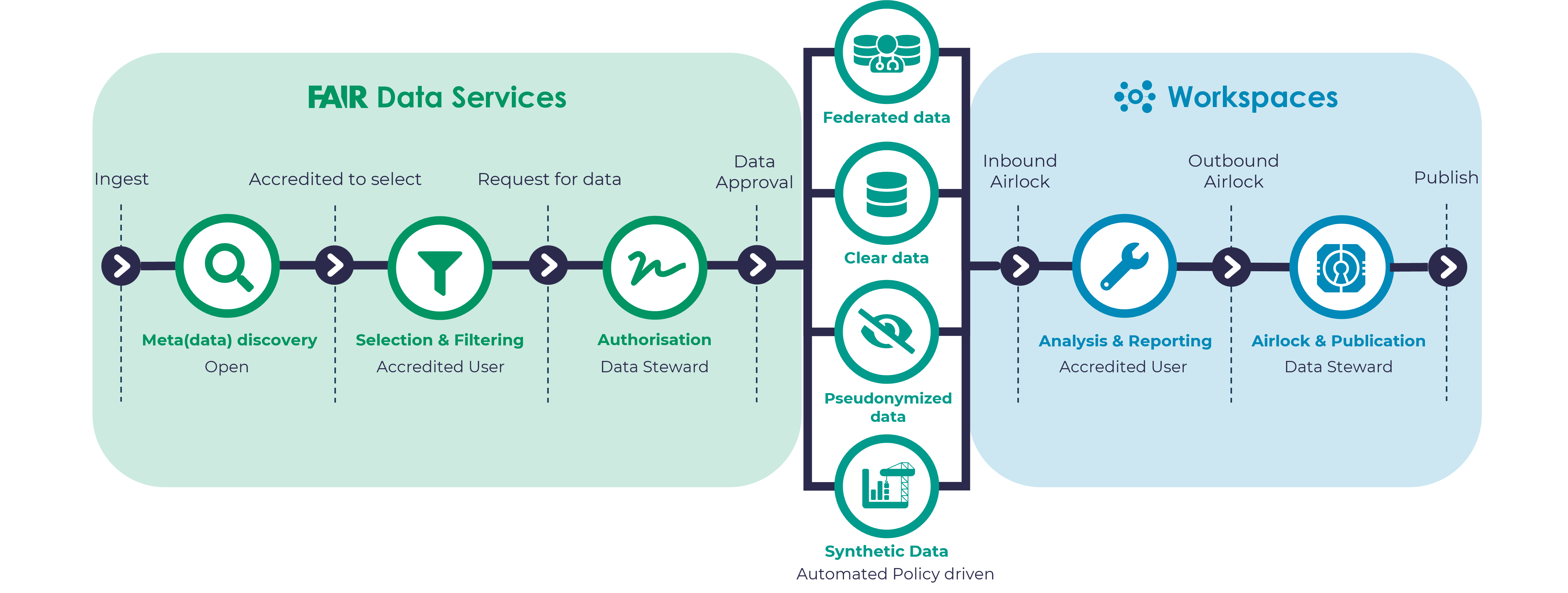
The Aridhia team worked closely with the grant PIs to brand their DRE training Hub proposal to not only ensure that the environment meets their needs, but to let their partners, collaborators, staff and their own ecosystem know that it is their environment owned and governed by the pediatric clinical pharmacology training community, principally the National Institute for Child Health and Development (NICHD). Aridhia’s early role is often to stand up an initial environment to illustrate the core functionality of the environment which, at its simplest, is the complement of FAIR and Workspaces services as illustrated in Figure 1 below.
One of the more unique aspects of the R25 training Hub proposal is the emphasis on growing a teaching faculty and external mentorship community which can be shared across the national and global pediatric clinical pharmacology ecosystem proving both didactic training to a community of trainees as well as 1:1 mentorship to individual trainees. This is facilitated by the DRE’s access and security features including the ability to invite colleagues into a training room (workspace) for a finite time window so that coding and methodology collaboration can happen in an environment with standard networking tools such as Teams and Zoom.
A part of the actual training and practicing certain approaches and modelling aspects will likely require data and/or code sharing. This too is easily managed as part of the workspace governance within the DRE. The data airlock allows the data steward (see Figure 1) to authorize the data request and upon approval provide it to the trainee (data customer) in their workspace. An added benefit of the system is the full audit trail of such transactions and the virus scan and data encryption features on data transfers.
The grant proposal is only a single example of how the DRE can be used for training purposes. Teaching environments for quantitative research in general typically involve a structured classroom setting with access to data analysis software, where students can actively practice applying quantitative methods to real-world datasets, often using hands-on exercises, group work, and discussions to solidify their understanding of concepts like statistical tests, data visualization, and interpretation of results; this can include both traditional in-person classrooms and online learning platforms with interactive features.
Quantitative Research Essentials
Key elements of a good quantitative research teaching environment include the following: Data access (providing students with access to relevant, real-world datasets, either from public sources or through research projects, to practice analysis skills on meaningful data); Software proficiency (adequate training in statistical software packages like SPSS, R, or SAS, allowing students to perform data cleaning, manipulation, and analysis independently); Interactive learning (incorporating activities like case studies, group projects, and simulations where students apply quantitative methods to solve problems and interpret results); Conceptual understanding (balancing technical skills with a strong foundation in the theoretical concepts behind quantitative research methods, including research design, hypothesis testing, and validity concerns); and Feedback and discussion (facilitating open discussion about the limitations of quantitative research, potential biases, and ethical considerations in data collection and analysis). These elements are easily appreciated within the Aridhia TRE environment and have been realized as part of the R25 grant application for the clinical pharmacology / pharmacometrics trainee community. Of course, other communities in the quantitative sciences would also benefit. Disciplines such as programming in general, bioinformatics and statistics as well as more specialized fields such as health economics, medical surveillance and epidemiology would similarly benefit.
Aridhia has great experience with a diversity of customers with very specific requirements that often change over time. Teaching quantitative sciences, particularly those that benefit from practical, hands-on exercises and either peer-reviewed or mentored oversight can really benefit from a trusted research environment that provides secure access to training materials of various file types (slide, document or video files, etc.) as well as a compute environment with various open-source or commercial software solutions where practice problems and coding solutions can be undertaken. The Aridhia Trusted Research Environment platform provides such an environment which can be entirely customized to support the specific training requirements needed.
March 10, 2025
Jeff Barrett
Dr. Jeff Barrett is the Chief Science Officer at Aridhia promoting healthcare and life science partners to collaborate, access and share secure data to deliver better patient outcomes. Before Aridhia, he was Senior Vice-President at the Critical Path Institute serving as the Executive Director of the Rare Disease Cures Accelerator, Data Analytics Platform. Jeff was previously Head of Quantitative Sciences at the Bill & Melinda Gates Medical Research Institute. Prior to MRI, he was Vice President, of Translational Informatics at Sanofi Pharmaceuticals. Jeff spent 10+ years at the University of Pennsylvania where he was Professor, Paediatrics and Director, Laboratory for Applied PK/PD at the Children’s Hospital of Philadelphia.