Blogs & News
RDCA-DAP and the Aridhia DRE – A platform to accelerate rare disease therapy development and regulatory engagement
Last week marked the first annual Rare and Orphan Disease Conference in Washington, DC which was attended by Aridhia’s Kara Lasater (Head of Customer Success) and Laura Shishodia (Product Manager). Critical Path Institute (C-Path) and the US Food and Drug Administration (FDA) hosted the conference and highlighted recent innovations and upcoming trends in rare disease drug development and regulatory engagement.
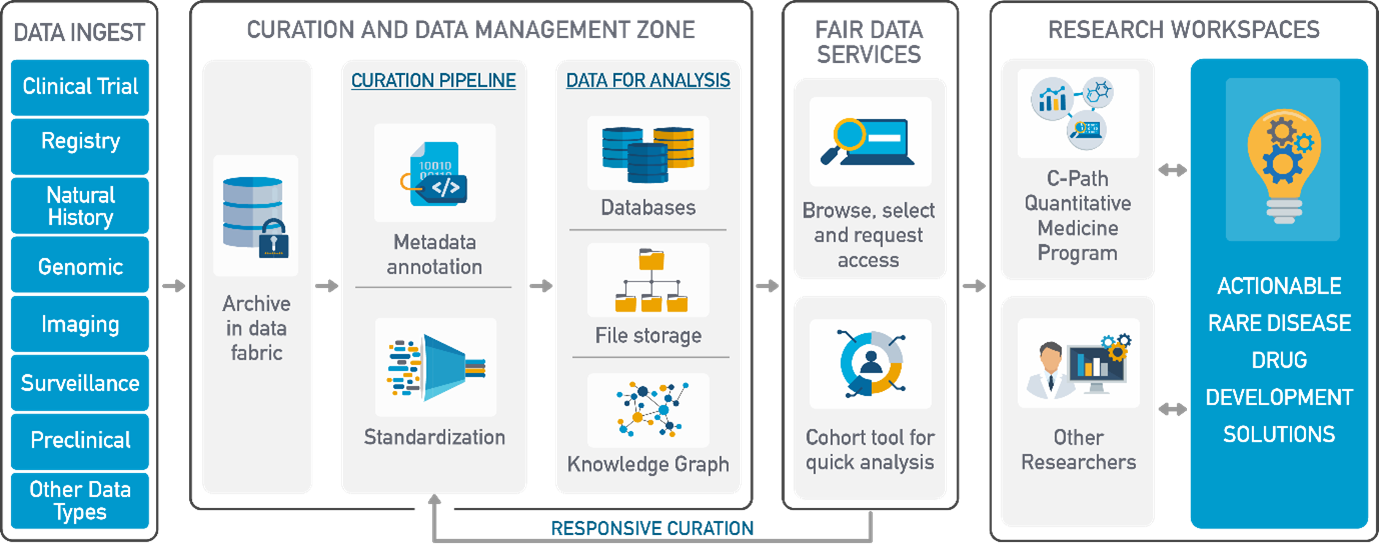
It also offered a firsthand look at the RDCA-DAP, powered by the Aridhia DRE, to a wide community of rare disease patients, providers, researchers, clinicians, biopharmaceutical companies, regulatory reviewers, and scientists. The conference offered a rare opportunity to share exciting progress and new approaches to drug development through the use of privileged data made discoverable and available through the RDCA-DAP/Aridhia DRE Platform.
Real-time data and analytics
Throughout the conference, biopharma, patient advocacy, and regulatory attendees discussed data sharing and collaboration on the platform and how it’s impacting drug development. Notably, how collaboration using RDCA-DAP is bridging the gap between patient and industry groups to share data more widely for better informed research and drug development.
All data contributors whether from patient advocacy groups, industry, or healthcare have control on how their data is discovered, accessed through permissions and used in studies and trials. The RDCA-DAP/Aridhia DRE brings a new level of transparency on data use that breaks down many of the current barriers to data collaboration and sharing; something that’s critical for rare diseases where no single organisation has sufficient power and quality within their own data repositories. If we want to see progress, we have to enable collaboration on data alongside legal and ethical governance on data use.
A key theme at the conference was the potential use of the RDCA-DAP/Aridhia DRE for regulatory engagement and submissions. Both patient advocacy groups and industry representatives were vocal in expressing a desire to see a more streamlined approach to regulatory engagement that used the data, analysis methods, code, and results from Workspaces projects as a run-in workflow to regulatory engagement. The opportunity for real-time collaboration directly with regulatory agencies by bringing together, data, code, tools, and results in the same environment now exists, enabling engagement with health authorities across various stages of research and clinical trials in real time.
Showcasing how the platform is currently supporting that collaboration, Laura and Kara showed key features of the platform in live demonstrations for ‘FAIR’ data sharing, making it easier to access data, and applying it to ongoing research and clinical trials. Additionally, they illustrated examples of dynamic data browsing and visualisations using the FAIR Data Services cohort builder and expanded on the advanced analytical platform tooling. Using Workspaces, they demonstrated statistical modelling applications, Jupyter Notebooks, and machine learning capabilities.
Novel approaches to clinical trials
With FDA initiatives like the Accelerating Rare Disease Cures (ARC) program and Operation Warp Speed for Rare Diseases, there is growing support for finding more efficient ways to evaluate drug submissions for rare diseases. With small patient populations and an often-limited understanding of disease progression posing significant challenges for clinical trials, rare diseases often struggle to meet the classic criteria for acceptance.
This gap was highlighted as a key area to address and was voiced frequently throughout the conference. Using the Aridhia DRE as the foundation for RDCA-DAP opens the door for platform trials and other adaptive trial designs. As the DRE facilitates the ingestion, curation and integration of disparate data types (e.g., registry data, natural history data, EHR data and data from clinical trials) patient trajectories can be created from these sources to evaluate disease progression and discern patient phenotypes allowing sponsors to refine patient selection criteria and ultimately design more informative trials with more meaningful clinical endpoints.
From the regulatory perspective, FDA discussed mounting openness to novel approaches such as adaptive trials and supplementing trial data with real world and natural history data in rare disease drug submissions. This more holistic approach is already being applied (e.g., Skyclarys 2023, Besremi 2021) and with more ‘FAIR’ data available, it paves the way for new pathways to drug approval.
In addition to recent successes, it’s also important to consider how failed trials can be invaluable for rare disease research. Where trials unfortunately prove to be ineffective, they can offer surprising insight as natural history datasets. With these validated as effectively placebo datasets, they could help trial sponsors move away from placebo-based trials for rare disease patients, ensuring more patience are given the opportunity to receive treatment.
Future directions for rare disease research
The conference reflected the growing appetite and optimism for using a modern data and analytics platform to work together to accelerate research and treatment development. When combined with a wide range of data spanning clinical trials natural histories, EHR, imaging, and omics, data barriers are lowered and more data can be combined to help accelerate drug development.
While FDA engagement was often at the centre of the conference, EMA engagement was also showcased; C-Path’s partnership with ERDERA is paving the way for new drug development tools (DDTs) across different disease areas and work with EATRIS is helping to identify drugs that could be repurposed for other rare diseases.
With FDA and EMA actively engaging on the platform, new opportunities are emerging for engagement with MHRA, PMDA, and other international health authorities. By leveraging diverse data from global industry and patient communities and regulatory engagement, researchers are utilising RDCA-DAP’s rich repository of data and combining with their own data and tools to conduct novel research and make a tangible impact on patients living with rare and orphan diseases.
September 22, 2023
Kara Lasater
Kara joined Aridhia in 2020 and leads the Customer Projects team as Head of Customer Success where she works closely with clients to deliver valuable solutions on the DRE. Kara leads with a commitment to understanding and addressing customer needs and fostering a customer-centric culture at Aridhia. In her free time, she enjoys hiking and taking road trips with her family and labradoodle, Wilson.